CORPORATION


Our Technology
Programmable Regenerative Medicine

We Are Coming
SOON
AIMER Nanotherapeutic Platform
(Active Immuno-Modulated Enhanced Regeneration)
The terms "AIMER", Activated Immuno-Modulated Enhanced Regeneration" and "Programmable Tissue Regeneration" are trademarks of Meridius Bio Corporation.

-
AIMER is a new nanotherapeutic technology that enhances wound healing strategies and products.
-
AIMER nanoparticles are topically applied or injected locally at the injury site. They are room temperature stable and have a very long (years) shelf life.
-
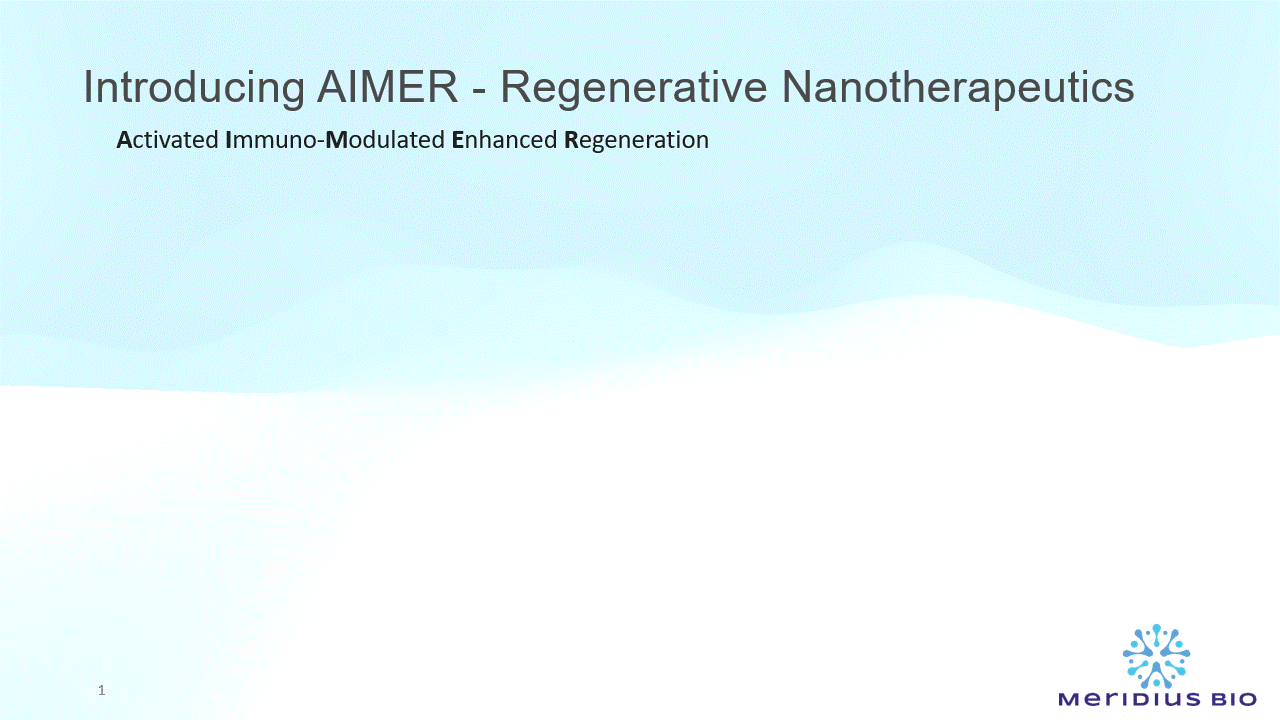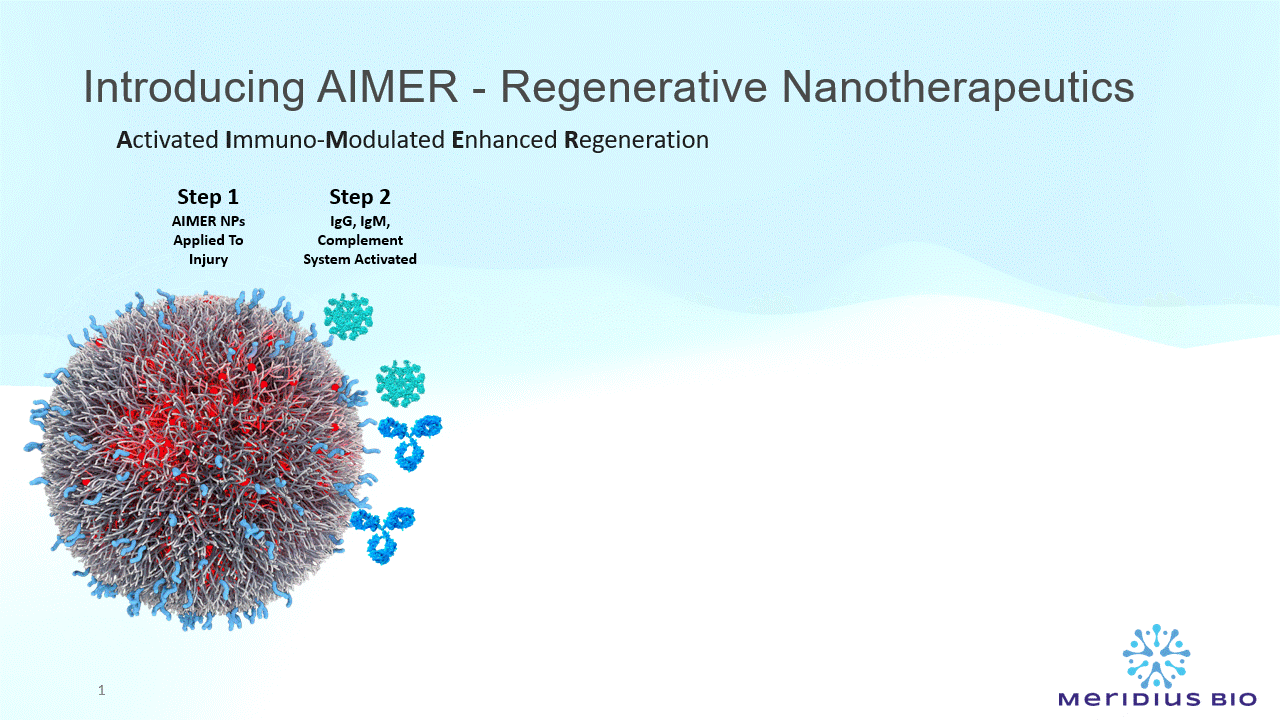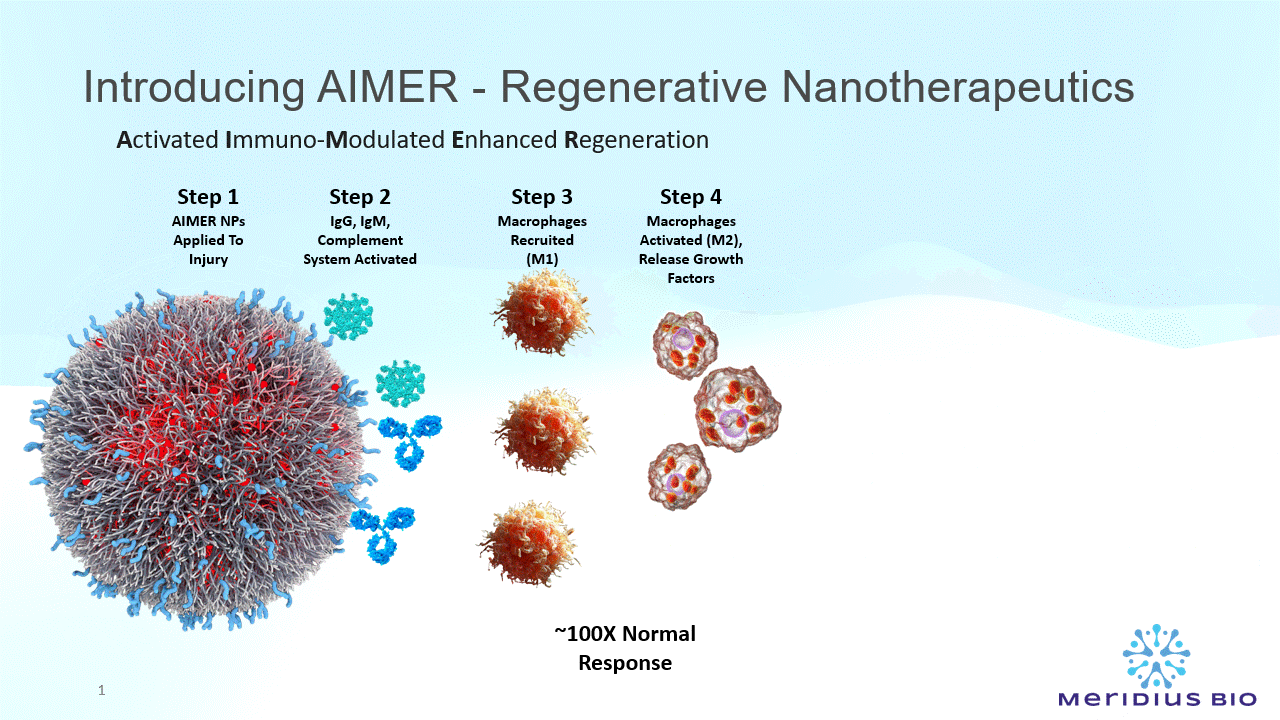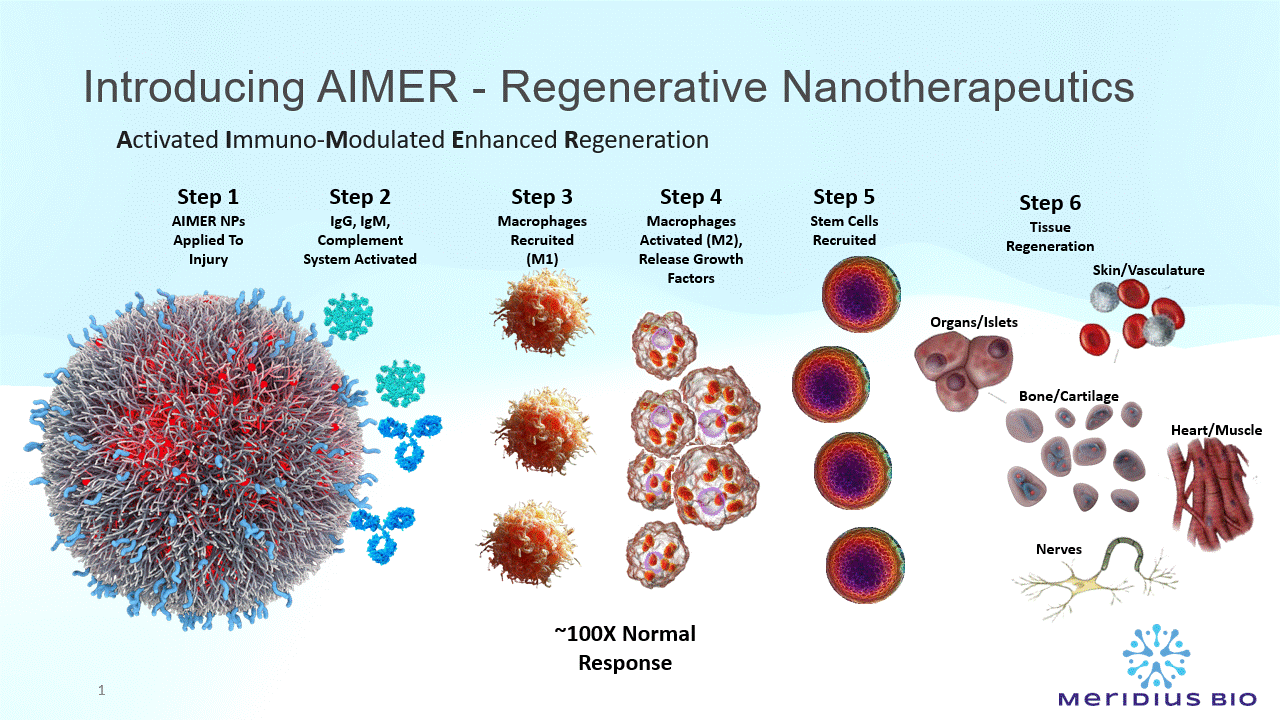
Animal model data has demonstrated 50-70% reduction in wound healing, concurrent regenerated normal tissue morphology, reductions in scarring and infections. This includes immune compromised animal models such as diabetes chronic ulcers and radiation burns.
-
Successful Proof-of-Concept in animal models for post-myocardial infarction regeneration, cruciate ligaments, CNS, spinal cord injury muscle and endocrine regeneration.
-
We have multiple issued patents for wound healing tissue regeneration applications using AIMER nanoparticles.

AIMER Technology
-
AIMER nanoparticles (NPs) comprise a special type of sugar molecule (Galactose-α-1,3-Galactose) significant in immune recognition, cell signaling, and pathogen-host interactions.
-
The AIMER NPs present multiple epitopes (recognition molecules) anchored on the nanoparticle wall.
-
There are ~10^14 epitopes per mg AIMER NPs.
-
AIMER NPs are applied locally, typically as a topical application. They are room temperature stable and have a very long (years) shelf life.
-
AIMER NPs can be produced from natural and synthetic sources.
Sign up to receive the first word when we go live.

Investment Risks & Mitigations
Safety & Toxicity - tested in Animal Models, in Humans:
-
Extensive supporting experimental data in Murine and Porcine species (alpha-1,3GT Knockout, human-like immune systems).
-
Additionally pre-clinical safety and toxicity studies have been done by others for a human cancer treatment. In December 2022, they completed a Phase I/IIa trial where it was reported that the study met its primary endpoint for safety and tolerability in humans. Positive secondary endpoint findings related to immune response and markers of clinical efficacy were also reported.
-
Regulatory Risks:
-
Given the extensive experimental and formal testing that has been completed, we believe the AIMER technology represents a significantly de-risked asset.
-
Clinical endpoints for Wound Healing are typically determined in days and weeks, not years.



